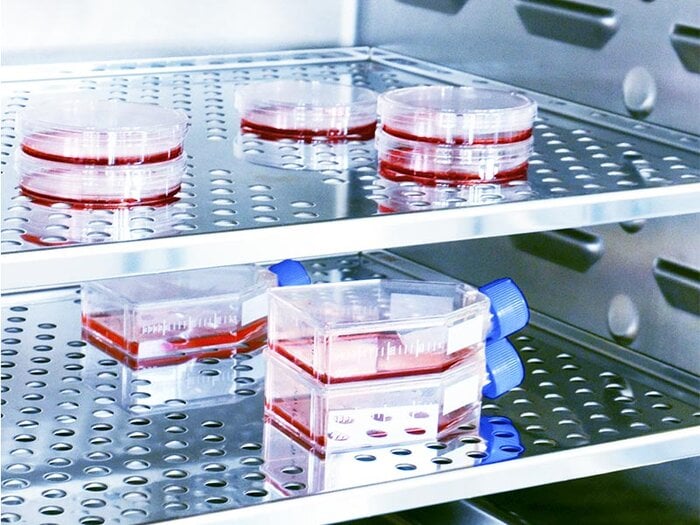
BINDER simulation chambers.
Always the right conditions.
At BINDER, we create best conditions for your success with climate chambers and incubators engineered and manufactured in Germany and trusted by scientific and industrial laboratories around the world.
Our mission is to make a meaningful technical contribution to human health and safety while protecting the environment. That’s why sustainability, accuracy and long-term value are central to every product we create - so you can focus fully on what drives you: research and development that shapes our future.
Worldwide trust in BINDER
From research labs to high-tech industries: BINDER chambers prove themselves every day. These selected case studies show how reliably and efficiently our solutions help customers meet tough challenges.
Meet BINDER live
More than just a job –
become part of the BINDER family

At BINDER, you’ll find a workplace defined by openness, team spirit, and genuine appreciation. As a family-owned company, we make sustainable, forward-thinking decisions and offer flat hierarchies with plenty of room for personal responsibility. Help shape the future with a team that truly sticks together.